Published by: Hubei Leizi
WUST News (Correspondent: Hubei Leizi) Recently, Chen Ao, a young teacher from the School of Life Science and Health at WUST, published a research paper entitled “Targeting the Oncogenic m6A Demethylase FTO Suppresses Tumorigenesis and Potentiates Immune Response in Hepatocellular Carcinoma” in the top international journal of hepatology Gut (IF 31.8, a top journal in Q1 of the Chinese Academy of Sciences) as the first author.
Liver cancer is the sixth most common cancer worldwide, causing approximately 830,000 deaths each year. Hepatocellular carcinoma (HCC) is the most common primary liver cancer, accounting for approximately 90% of liver cancer cases. HCC has hidden early symptoms and is usually diagnosed at an advanced stage of liver cirrhosis, with very limited clinical treatment effects and poor prognosis for patients. In addition, traditional treatments such as surgical intervention, radiotherapy, and chemotherapy are non-specific, and may not effectively prolong survival while causing significant harm to patients. However, tumor immunotherapy has unique advantages in terms of tumor tissue specificity and precision. This study hopes to release effector T cells to fight tumors by stimulating or removing the suppression of the immune system. It combines the advantages of chemotherapy and immunotherapy to further improve the therapeutic effect.
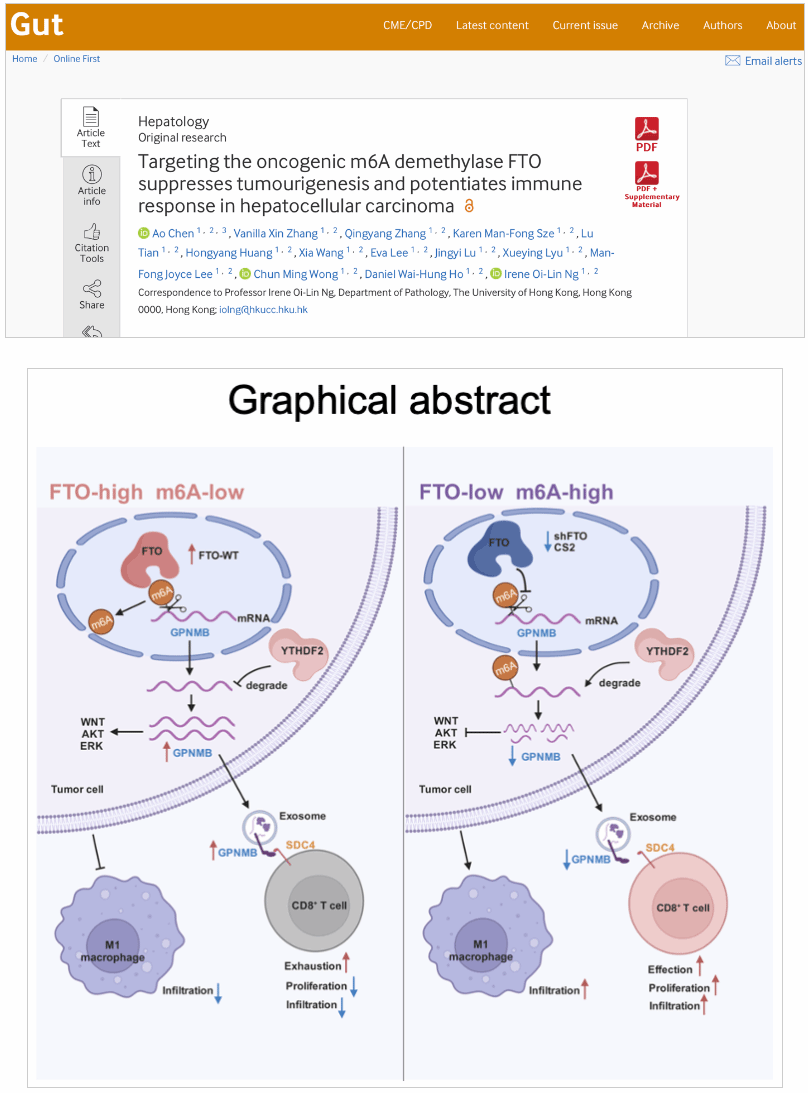
Fat mass and obesity-associated protein (FTO), an N6-methyladenosine (m6A) demethylase, plays an oncogenic role in various cell types by "deleting" m6A marks on mRNA. Extracellular vesicles (sEVs, or exosomes) are critical mediators of tumor genesis and metastasis. However, the interplay between FTO-mediated m6A modification and sEVs in hepatocellular carcinoma (HCC) remains elusive.
This study investigated the role of FTO in HCC. We found that FTO expression was significantly upregulated in HCC patient samples. In vitro experiments showed that FTO knockdown significantly suppressed HCC cell proliferation, migration, and invasion. In vivo, FTO knockdown significantly inhibited tumor growth and lung metastasis. Furthermore, FTO knockdown enhanced CD8+ T cell activation and recruitment and M1 macrophage polarization in C57BL/6 mice. Transcriptome and m6A sequencing identified GPNMB as a downstream target gene of FTO-mediated m6A modification, and this process was mediated by YTHDF2-induced GPNMB mRNA degradation. Immunoprecipitation and immunogold experiments confirmed that GPNMB protein localized to the exosome surface directly binds to the CD8+ T cell surface receptor protein SDC4 and suppresses its activation and recruitment. In conclusion, FTO downregulates m6A levels on the mRNA of its downstream target gene GPNMB, and subsequently suppresses exosome-mediated immune cell activation and recruitment, ultimately promoting HCC progression.
Dr. Chen Ao was selected for the Hong Kong Scholars Program in 2020, jointly funded by the National Postdoctoral Management Committee and the Society of Hong Kong Scholars. He joined the laboratory of Prof. Irene Oi-Lin NG, Director of the State Key Laboratory in the Department of Medicine at the University of Hong Kong, to conduct scientific research. During this period, Dr. Chen Ao's research on the mechanisms of epigenetic regulation in the development and progression of HCC is one of the hot research topics in the field of liver cancer. Therefore, the publication of this research result is an important advance in this hot research topic of liver cancer and a collaborative work to deepen scientific research cooperation between the Chinese mainland and Hong Kong.