Published by: Zhao Yabin Edited by: Zhou Tongjiang
WUST News (Correspondent: Zhao Yabin) – A research team led by Professors Huo Kaifu and Zheng Yang from the School of Metallurgy and Energy at the Wuhan University of Science and Technology (WUST) published significant research findings in Advanced Functional Materials (Impact Factor 19.0; CAS 1st District TOP), a renowned journal in the field of materials science. The paper, titled Competitive Solvation Chemistry Modulated Nonflammable Pseudo Ultralow Concentration Electrolyte Toward High‐Voltage Li Metal Batteries, highlights a novel approach to improving the safety and performance of lithium metal batteries. Professor Zheng Yang, Distinguished Professor Li Zhuo, and Professor Huo Kaifu are the corresponding authors of the paper, with WUST listed as the primary institution.
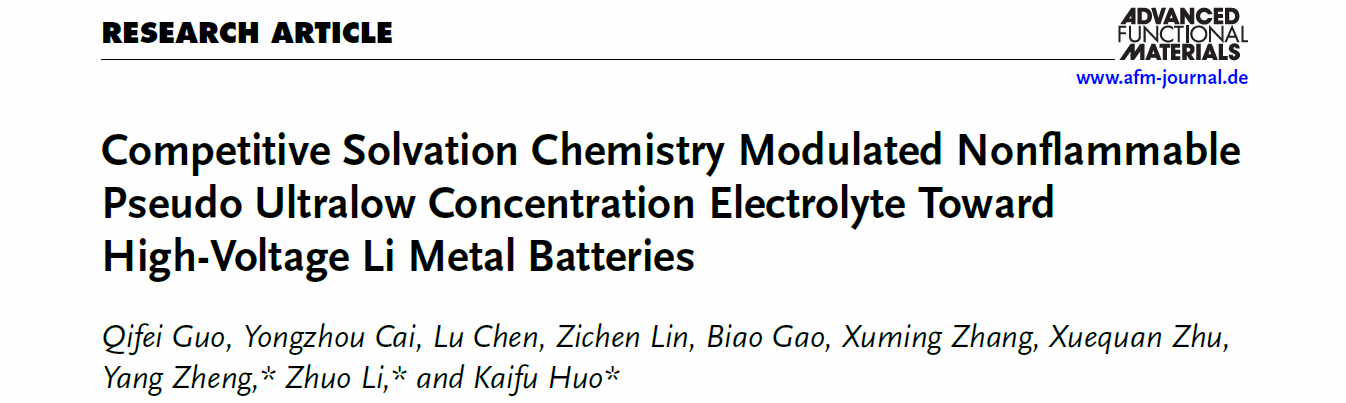
Lithium metal batteries are considered a promising candidate for future energy storage technologies due to their extremely high theoretical energy density. However, issues such as uncontrollable lithium dendrite growth in commercial electrolytes lead to poor cycling performance and severe safety concerns, significantly hindering their application. To address these challenges, the research team has innovatively proposed a "pseudo ultralow concentration electrolyte" design. Based on a competitive solvation regulation mechanism, they developed a novel, highly safe, low-concentration electrolyte, which significantly improves the electrochemical performance and safety of lithium metal batteries. This work offers valuable new perspectives on advanced electrolyte design for lithium metal batteries.
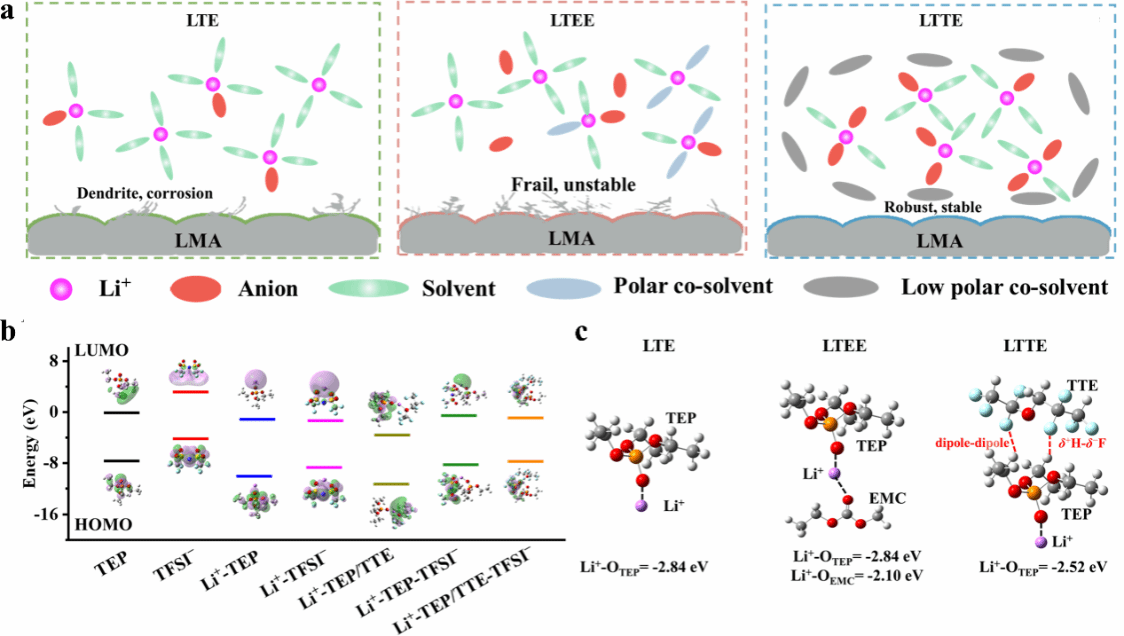
The research team designed a pseudo ultralow concentration electrolyte with high ionic conductivity, flame retardancy, and a wide electrochemical window by incorporating a high proportion of fluorinated ether inert co-solvent to a phosphate-based active solvent. The dipole-dipole interaction between the fluorinated ether and phosphate molecules triggers a competitive solvation effect among the co-solvent, phosphate, and Li+, effectively modulating the solvation structure of Li+ and enhancing electrode interface stability. The Li||NCM622 pouch cell based on this electrolyte exhibits excellent cycling stability.
Paper link:
https://advanced.onlinelibrary.wiley.com/doi/abs/10.1002/adfm.202500500