Published by: Luo Shi | Edited by: Wang Xiangyu
WUST News (Correspondent: Luo Shi) — Researchers from the School of Metallurgy and Energy at Wuhan University of Science and Technology (WUST), in collaboration with Huazhong University of Science and Technology and Tsinghua Shenzhen International Graduate School, have published a significant study titled Direct Construction of a LiF-Rich Interphase for Sustainable Regeneration of Spent Graphite Electrodes via In Situ Decarbonization-Fluorination Strategy (DOI: 10.1002/adma.202514869) in the internationally renowned journal Advanced Materials (Impact Factor: 26.8; Chinese Academy of Sciences Top Tier, Category 1). Professors Gao Biao and Huo Kaifu from WUST's School of Metallurgy and Energy, together with Professor Zhou Guangmin from Tsinghua Shenzhen International Graduate School, served as corresponding authors. Doctoral candidates Luo Shi and Liu Fengrui are the co-first authors, with WUST as the primary institution.
The recycling of spent lithium-ion batteries is a hot issue in the fields of environmental and energy materials. Regenerating graphite anodes from end-of-life batteries offers a promising route to alleviate resource scarcity and mitigate environmental pollution. While conventional pyrometallurgical and hydrometallurgical methods have seen initial deployment, they often suffer from low regeneration efficiency, high processing costs, and severe secondary pollution.
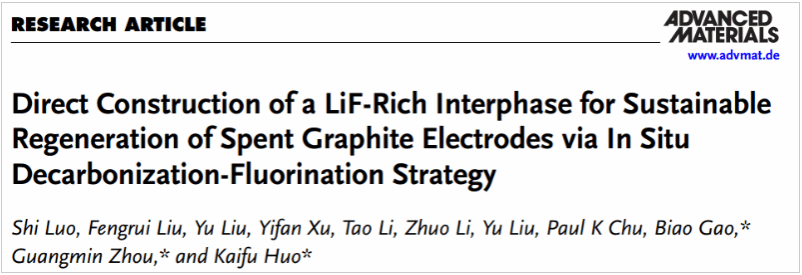
This study introduces a decarbonization–fluorination strategy using an aqueous NH₄F solution to directly convert unstable, low-conductivity Li₂CO₃ on the surface of spent graphite into a highly stable, ionically conductive LiF-rich interphase. This enables direct electrode regeneration without requiring delamination or powder separation from the current collector. The reconstructed interface significantly enhances ionic conductivity, reduces interfacial resistance, and establishes fast Li⁺ transport pathways. The regenerated graphite anode delivers a specific capacity of 303.9 mAh g⁻¹ at 0.5 C, comparable to commercial graphite.
When paired with an NCM811 cathode in a 550 mAh full-cell configuration, the regenerated anode retained 92% capacity after 200 cycles at 1 C, with an areal capacity 4.9 times higher than that of cells using untreated spent graphite electrodes. Techno-economic analysis indicates that, this method eliminates the need to delaminate "black mass" from the current collector and avoids highly polluting, energy-intensive processes such as acid leaching or high-temperature treatment, reducing costs by approximately 78%. The approach demonstrates strong potential for practical industrial application.
This study presents a decarbonization–fluorination-based interfacial reconstruction strategy that enables direct recycling of spent graphite anodes, significantly streamlining the regeneration process, cutting costs and emissions, and offering a novel pathway toward green, low-carbon recovery of spent graphite anodes.